Precision Liquid Dispensing: The Key to Faster, Scalable Diagnostic Development

The need for rapid, reliable, and scalable diagnostic tests has never been greater, whether it’s for addressing challenges with SARS-CoV-2 infections or detecting sexually transmitted diseases like HIV and other pathogens. To meet these needs, manufacturers are turning to precision liquid microdispensing technology, which enables miniaturization of multiplex assays, exceptional accuracy, and scalable high-throughput production. This innovation is transforming diagnostic manufacturing by improving efficiency, reducing waste, and ensuring consistent test performance. In this article, we explore how precision microdispensing is shaping the future of diagnostics, making advanced healthcare solutions more accessible, cost-effective, and impactful worldwide.
How Precision Dispensing is Transforming Diagnostic Manufacturing
Meeting the Demand for Diagnostic Tools
The global demand for rapid, cost-effective diagnostic tests continues to grow, driven by the need for accessible, locally deployable, point-of-care tests [1]. The COVID-19 pandemic underscored the urgent need for advanced testing infrastructure and highlighted the importance of rapidly and reliably detecting pathogens [2]. Additionally, there remains a critical demand for diagnostic tools to assess food and water safety, such as microbes in drinking water [3,4].
To meet these challenges, diagnostic tools must offer the highest sensitivity on one hand and cost-efficiency on the other. However, their development requires cutting-edge manufacturing technologies that ensure accuracy, reliability, and scalability.
For example, to address the need for precancer screening in cervical cancer, researchers used the sciFLEXARRAYER S3 from SCIENION to develop a point-of-care test for cancer-associated proteins. Due to its low production costs, this test might enable low- and middle-income countries to improve and extend diagnostic testing procedures, as the high costs and usability challenges of the current healthcare system have previously been significant barriers [5].
As the demand for such tests increases, so does the need for advanced production techniques to create smaller, more efficient, and highly sensitive diagnostic solutions [6].
Precision Dispensing: Advanced Solutions for Today’s Challenges
The modern medical landscape increasingly relies on point-of-care testing solutions, particularly in the wake of the COVID-19 pandemic, where diagnosis in the domestic setting reduced the strain on healthcare infrastructure [7]. Advanced precision dispensing at micro-, nano-, and picoliter scales might open new possibilities for developing next-generation diagnostic tools. By leveraging automation and technologies like the Piezo dispense capillary (PDC) based sciDROP PICO, these capabilities enhance accuracy and quality.
Recently, researchers used SCIENION technology to create a multiplex assay for SARS-CoV-2, allowing them to observe how individuals’ serum antibody profiles varied depending on their exposure to different viral variants and their vaccination status (Fig. 1) [8]. This involved the dispensing of viral antigens and control protein in 150–200-μm spots on reflective phantom interface (RPI) sensing chips.
Recent dispensing innovations ensure precise fluid handling, enabling the miniaturization of tests without compromising their performance, ultimately driving advancements in global healthcare diagnostics while reducing the amount of sample and expensive reagents required per test [6].
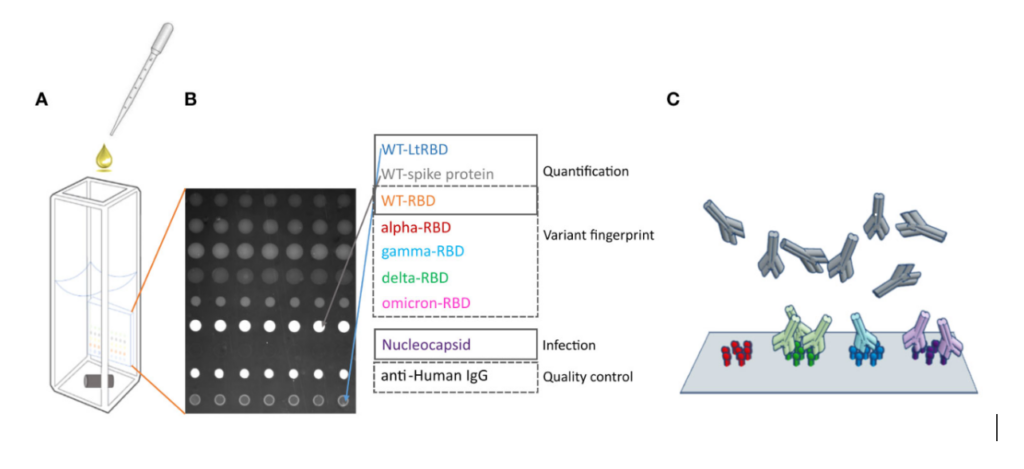
Figure 1. SCIENION’s technologies helped researchers develop an assay to detect serum antibodies targeting different SARS-CoV-2 antigens.
Adapted from:
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1323406/full
From R&D to Implementation – The Eurostars E*LOGIC Project
The Challenge:
Monitoring antibiotic residues in food is a critical requirement to ensure consumer safety and comply with stringent global regulations [9]. To address this challenge, the Eurostars project E*LOGIC was launched to develop a rapid, multiplex immunodiagnostic test capable of detecting seven key antibiotic targets across four major drug families (Nitrofurans, Tetracyclines, Nitroimidazoles, and Chloramphenicol) in the seafood and honey industries.
The Solution:
SCIENION played a pivotal role in developing this diagnostic tool by leveraging its cutting-edge sciFLEXARRAYER technology. The non-contact dispensing system enabled precise microarray printing of binding ligands on nitrocellulose membranes, ensuring high sensitivity and reliability. Carbon nanoparticles were integrated as detection labels, further enhancing accuracy. SCIENION also contributed to scaling up production by optimizing test parameters, including membrane pore size, reagent concentrations, and automated quality control.
The Impact:
The resulting prototype kit allows rapid detection within 10 minutes, significantly reducing testing time and costs for seafood and honey producers, government laboratories, and regulatory bodies.
Learn more about the E*LOGIC Project and other success stories.
Key Advantages of Precision Liquid Dispensing in Diagnostics
Microdispensing comes with several benefits for the production and development of diagnostic tools. Primary advantages include:
Ultra-Low Volume Dispensing
Ultra-low volume dispensing allows researchers to use only a minimal amount of reagents when developing and producing diagnostic tests. This approach reduces waste and optimizes resource efficiency. Additionally, when paired with a dispensing technology like the sciDROP PICO from SCIENION, researchers can achieve even greater efficiency thanks to its zero dead volume capability.
High-Throughput Automation
Automation offers a wide range of benefits, including faster processing and reduced errors commonly associated with manual handling and dispensing [10]. High-throughput automated microdispensing capabilities enable significantly faster processing, making it ideal for the rapid, large-scale production of diagnostic tests—an essential factor in responding to emerging healthcare emergencies, such as the COVID pandemic, where millions, if not billions, of lateral flow tests were required almost overnight.
Precision and Consistency
Advanced microdispensers enable precise liquid dispensing at volumes as low as 10 pL. They also enhance assay performance by executing consistent and programmable dispensing tasks without manual intervention. This ensures that assays can be developed with precision and uniformity across multiple geographic locations, delivering reliable and reproducible results that meet regulatory requirements and quality standards.
Flexibility
Flexibility is a key advantage throughout the transition from development to full-scale manufacturing. It gives researchers the flexibility to make adjustments early in the process without significant losses in time and resources, which would be more costly at later stages. Once manufacturing is optimized, processes can be quickly scaled up while still using minimal reagent quantities, ensuring cost efficiency even in large-scale production. sciFLEXARRAYER instruments from SCIENION provide dispensing solutions for all scales, from exploratory work and batch printing to high-throughput manufacturing. This flexibility sets SCIENION’s offerings apart from competitors.
How Scienion’s Technology Enables High-Performance Diagnostics
Picoliter Dispensing and Nanoliter Pipetting for Miniaturization and Accuracy
Picoliter dispensing enables the miniaturization of diagnostic production and development. Smaller droplet sizes, compared to other dispensing methods, allow for multiplexing of assays using smaller devices while increasing the density of analytical components within a single unit. This leads to reduced raw material consumption and improved throughput.
For precise and reproducible nanoliter dispensing, SCIENION’s sciDROP NANO technology offers a reliable solution. It delivers dispensing precision with a coefficient of variation of less than 2%, ensuring uniform assay reagent deposition for more accurate testing and successful product development.
SCIENION vs. the Competition
SCIENION provides researchers with highly flexible and efficient dispensing solutions across various applications. The sciFLEXARRAYER range offers precise control over sample deposition, supporting both full-line and segmented-line printing to optimize resource usage and minimize waste. The instruments support the customer at all phases, from development to mass production. For greater scalability, the sciDROP NANO and sciDROP PICO technologies seamlessly integrate with the sciFLEXARRAYER platform, enabling rapid printing and high-throughput production (Fig. 2).
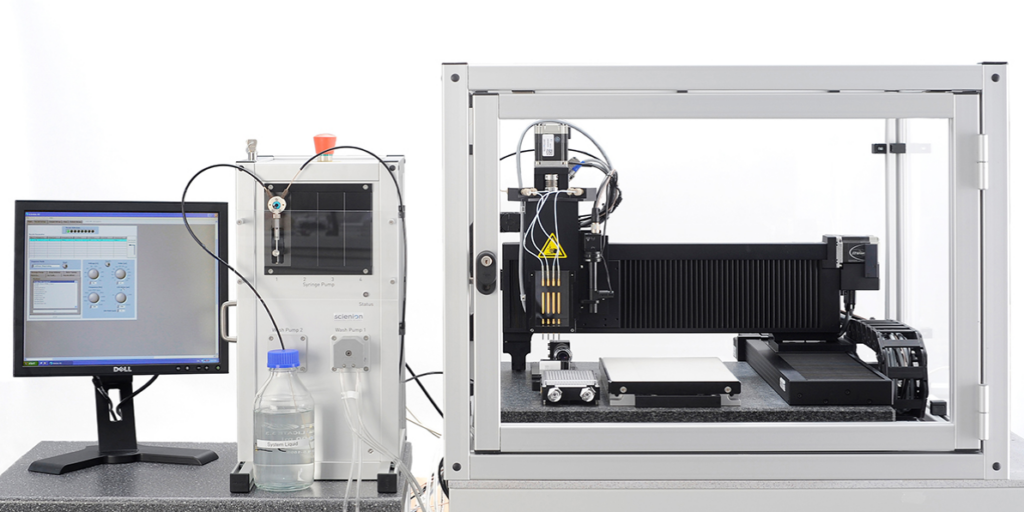
Figure 2. sciFLEXARRAYER platform, an R&D entry platform, enables rapid and reproducible diagnostic assays.
With dispensing capabilities as low as 10 pL, SCIENION surpasses competitors, delivering greater precision and enhanced miniaturization. Additionally, SCIENION’s dispensing capillaries, handmade and QC’d in our Berlin facilities, can be coated with different solutions, enabling the dispensing of virtually any type of liquids, aqueous or organic, with minimal retention. SCIENION offers on-site support to help researchers fully leverage its dispensing technology, along with a comprehensive range of services, from Contract Development to Contract Manufacturing, enabling both researchers and diagnostics manufacturers to streamline their workflows and achieve cost and time savings.
The Future of Diagnostic Test Manufacturing
Precision liquid microdispensing is transforming diagnostic manufacturing, making it possible for companies to produce high-performance, scalable, and cost-effective tests with exceptional accuracy. SCIENION is at the forefront of this innovation, providing cutting-edge dispensing solutions that enhance efficiency, reduce waste, and ensure consistent test performance. As the demand for advanced diagnostics grows, manufacturers must embrace precision dispenser technology to stay competitive.
Partner with SCIENION to accelerate your diagnostic development and bring next-generation healthcare solutions to market faster than ever.
References
- Dinnes J, Sharma P, Berhane S, van Wyk SS, Nyaaba N, Domen J, et al. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705.
- Harpaldas H, Arumugam S, Campillo Rodriguez C, Kumar BA, Shi V, Sia SK. Point-of-care diagnostics: recent developments in a pandemic age. Lab Chip. 2021 Nov 25;21(23):4517–48.
- CDC. Guidelines for Testing Well Water [Internet]. Drinking Water. 2024 [cited 2025 Mar 18]. Available from: https://www.cdc.gov/drinking-water/safety/guidelines-for-testing-well-water.html
- Arsenic [Internet]. [cited 2025 Mar 18]. Available from: https://www.who.int/news-room/fact-sheets/detail/arsenic
- Smith CA, Paul S, Haney KE, Parra SG, Bond M, López L, et al. A paper-based HPV E7 oncoprotein assay for cervical precancer detection at the point of care. Sci Rep. 2025 Jan 24;15(1):3041.
- Siavashy S, Soltani M, Rahimi S, Hosseinali M, Guilandokht Z, Raahemifar K. Recent advancements in microfluidic-based biosensors for detection of genes and proteins: Applications and techniques. Biosensors and Bioelectronics: X. 2024 Aug;19:100489.
- Khan AR, Hussain WL, Shum HC, Hassan SU. Point-of-care testing: a critical analysis of the market and future trends. Front Lab Chip Technol. 2024 May 13;3:1394752.
- Carzaniga T, Casiraghi L, Nava G, Zanchetta G, Inzani T, Chiari M, et al. Serum antibody fingerprinting of SARS-CoV-2 variants in infected and vaccinated subjects by label-free microarray biosensor. Front Immunol. 2024 Feb 27;15:1323406.
- Izah SC, Nurmakhanova A, Ogwu MC, Toktarbay Z, Umirbaeva Z, Ussen K, et al Public Health Risks Associated with Antibiotic Residues in Poultry Food Products. Journal of Agriculture and Food Research. 2025 Mar;101815.
- Holland I, Davies JA. Automation in the Life Science Research Laboratory. Front Bioeng Biotechnol. 2020;8(571777).