Microneedles – tiny devices with huge potential in drug delivery and POC diagnostics

Microneedles have the potential to revolutionize the healthcare industry in drug delivery and diagnostics. These microscopic needles, too small to be seen by the naked eye, have been designed to deliver drugs through the skin without causing pain. Compared to traditional methods like injections or oral medication, microneedles offer a range of benefits. They are minimally invasive, and can even be used to detect clinically significant substances through the skin. This blog post will delve into the world of microneedles, exploring their potential, benefits, applications, and challenges, and how they are poised to revolutionize drug delivery and diagnostics.
Introduction
Microneedles are very tiny, microscopic needles, generally between 50 and 900 µm in length. They were initially proposed as a painless and non-invasive method of drug delivery back in 1976 (1). Microneedle-based devices are manufactured using microfabrication techniques and consist of sharp protrusions in arrays. While the earliest versions were made from silicon, metal, or glass, recent models are constructed from biodegradable and hydrogel-based polymers. In 1998, Mark Prausnitz at the Georgia Institute of Technology illustrated that microneedles are suitable for transdermal drug delivery by penetrating the uppermost layer of human skin (2).
Types of Microneedles
Numerous types of microneedles are currently available in the market. Figure 1 showcases the different designs of microneedles that have been developed for diverse applications.
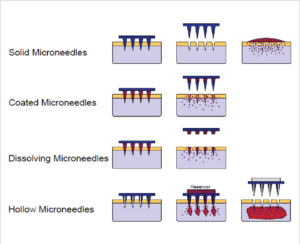
Figure 1: Schematic illustration of methods of microneedle application to the skin for drug delivery purposes (Figure adapted from Figure 1 in Ref.(15))
Solid microneedles work by penetrating the skin. Once removed they leave micropores in which drugs can be applied via a transdermal patch.
Coated microneedles have drugs applied directly to the needle array instead of using patches or applicators. Once hydrated by body fluids, the drug is released into the skin.
Dissolving microneedles contain drugs in a biodegradable polymer that dissolves inside the skin.
Hollow microneedles have reservoirs that allow drugs to be delivered through diffusion or pressure.
Hydrogel-forming microneedles contain a drug reservoir and a swelling material that diffuses the drug by absorbing the interstitial fluid through the swollen matrix.
Microneedle Applications
Microneedles have a variety of applications, such as:
Drug and vaccine delivery
Insulin delivery for diabetes management
Insulin delivery for diabetes management is a widely researched application (3). Traditional methods of multiple daily injections can be painful and inconvenient. Microneedles, on the other hand, can provide a painless and potentially more effective alternative for insulin administration.
Vaccination programs
Microneedles are also being investigated for the delivery of vaccines (4). They can deliver vaccines directly to the immune-rich layers of the skin, potentially enhancing the immune response. Additionally, their ease of use and painless nature make them an attractive option for mass vaccination programs.
Local anesthesia administration
Microneedles have been successfully used for the administration of local anesthesia (5). They can deliver the anesthetic directly to the desired area, providing quick and effective numbing without the pain associated with traditional needles.
Microneedling (cosmetics)
Microneedling has gained popularity in the cosmetic industry (6). In 2006 the first Microneedle device became available on the market developed by Dr. Desmond Fernandes (7), the device became the modern-day Dermaroller. The primary use for this device is to utilize solid microneedles for better skin care product penetration, such as serums that encourage collagen production, helping to remove scarring.
Biosensing and Point of Care Diagnostics
Microneedles can be used for minimally invasive sampling of interstitial fluid or biomarkers present in the skin. These biomarkers can be analyzed for diagnostic purposes, such as monitoring glucose levels for diabetes management or detecting other analytes.
Benefits of Microneedles
The use of microneedles offers numerous advantages, both from a patient and a therapeutic perspective.
Reduced pain and discomfort
Microneedles are an excellent option for patients who require frequent injections but are afraid of needles. They are so tiny that they can penetrate the skin without causing any pain. Research has shown that they can reduce pain by up to 90% compared to traditional hypodermic needles (8).
Increased drug absorption
Microneedles penetrate the skin’s outer barrier and deliver drugs directly to the dermal layer. This layer is rich in blood vessels, enabling faster and more efficient drug absorption. Moreover, drug delivery via microneedles avoids first-pass metabolism, which can reduce the effectiveness of certain drugs.
Less side-effects
The microneedle patch delivery method targets the skin’s immune system, resulting in a stronger immune response with a lower vaccine dose. This leads to more efficient use of the antigen (9).
Simple, independent self-treatment
Unlike traditional hypodermic needles, which can cause accidental needle injuries, contamination risks from multiple use, and intentional misuse, microneedle devices in the form of inexpensive disposable patches have the potential to be used safely without clinical expertise.
Challenges and Future Directions in Microneedle Drug Delivery
Despite the significant progress of microneedles, there are still several challenges to overcome before they can be widely adopted.
Safety and regulatory concerns
Firstly, safety and regulatory concerns need to be addressed. While microneedles are generally considered safe, ongoing research is needed to evaluate their long-term safety, particularly when used for prolonged periods. Furthermore, clear regulatory guidelines need to be established to ensure the quality and safety of microneedle products.
Scalability and cost-effectiveness
Another challenge lies in the scalability and cost-effectiveness of microneedle production. Current manufacturing processes can be complex and expensive, which may limit their widespread use. Efforts are underway to develop scalable and cost-effective fabrication methods, such as photolithography and 3D printing (10), to overcome this challenge.
Stability and precise coating of microneedles
To ensure the effectiveness of microneedles, they must remain mechanically stable throughout storage, handling, and application. Coating the microneedles with drugs, vaccines, or other biologicals is critical to maintain their functionality, but can present problems such as heating of carbohydrates and polymers, potentially leading to drug breakdown during molding at high temperatures (11). Additionally, precise coating of the microneedles and controlling the delivery of therapeutic substances is a challenge. Researchers are working towards improving the stability of therapeutic substances and optimizing the needles themselves (12), as well as enhancing the accuracy and uniformity of microneedle coating.
Coating of microneedles with SCIENION micro dispensing technology
There are different methods to coat microneedles, including dipping, rolling or brushing, spraying, and electrostatic coating (13).
Recent work from the Tyndall National Institute (14) has investigated the adaptation of SCIENION’s micro dispensing technology as a rapid, precise and effective microneedle coating method. The method demonstrated exceptional precision in applying formulations onto the microneedle surface. Additionally, the piezoelectric inkjet system presents promising prospects for mass production, taking into account factors such as scalability, production speed, and cost-effectiveness.
Conclusion
Microneedles have the potential to revolutionize the field of drug delivery and diagnostics, offering a painless and efficient method of administering drugs through the skin. Advantages, including reduced pain, increased drug absorption, and improved patient compliance, make them a promising alternative to traditional injection methods. Ongoing research and development efforts are focused on addressing the challenges associated with microneedle technology, such as safety concerns, cost-effectiveness and stability. With continued technological advancements such as inkjet coating, microneedles have the potential to transform the way we deliver drugs, opening up new possibilities for personalized medicine and improving patient outcomes.
References:
(1) Gerstel, M. S., & Place, V. A. (1976). Drug Delivery Device. Google Patents. US Patent No. US3964482A.
(2) Mark Prausnitz | School of Chemical and Biomolecular Engineering (gatech.edu)
(3) Jana, B. A., & Wadhwani, A. D. (2019). Microneedle – Future prospect for efficient drug delivery in diabetes management. Indian journal of pharmacology, 51(1), 4–10. https://doi.org/10.4103/ijp.IJP_16_18
(4) Sheng, T., Luo, B., Zhang, W., Ge, X., Yu, J., Zhang, Y., & Gu, Z. (2021). Microneedle-Mediated Vaccination: Innovation and Translation. Advanced drug delivery reviews, 179, 113919. https://doi.org/10.1016/j.addr.2021.113919
(5) Lee, B. M., Lee, C., Lahiji, S. F., Jung, U. W., Chung, G., & Jung, H. (2020). Dissolving Microneedles for Rapid and Painless Local Anesthesia. Pharmaceutics, 12(4), 366. https://doi.org/10.3390/pharmaceutics12040366
(6) Doddaballapur S. (2009). Microneedling with dermaroller. Journal of cutaneous and aesthetic surgery, 2(2), 110–111. https://doi.org/10.4103/0974-2077.58529
(7) Fernandes, D. (2005). Minimally invasive percutaneous collagen induction. Oral and Maxillofacial Surgery Clinics, 17(1), 51-63.
(8) Gill, H. S., Denson, D. D., Burris, B. A., & Prausnitz, M. R. (2008). Effect of microneedle design on pain in human volunteers. The Clinical journal of pain, 24(7), 585–594. https://doi.org/10.1097/AJP.0b013e31816778f9
(9) Vrdoljak A. Review of recent literature on microneedle vaccine delivery technologies. Vaccine: Development and Therapy. 2013;3:47-55
https://doi.org/10.2147/VDT.S34682
(10) Gülçür, M., Romano, J. M., Penchev, P., Gough, T., Brown, E., Dimov, S., & Whiteside, B. (2021). A cost-effective process chain for thermoplastic microneedle manufacture combining laser micro-machining and micro-injection moulding. CIRP Journal of Manufacturing Science and Technology, 32, 311-321.
(11) Donnelly, R. F., Morrow, D. I., Singh, T. R., Migalska, K., McCarron, P. A., O’Mahony, C., & Woolfson, A. D. (2009). Processing difficulties and instability of carbohydrate microneedle arrays. Drug development and industrial pharmacy, 35(10), 1242–1254. https://doi.org/10.1080/03639040902882280
(12) Lee, J. W., Park, J. H., & Prausnitz, M. R. (2008). Dissolving microneedles for transdermal drug delivery. Biomaterials, 29(13), 2113–2124. https://doi.org/10.1016/j.biomaterials.2007.12.048
(13) Ingrole, R. S. J., & Gill, H. S. (2019). Microneedle Coating Methods: A Review with a Perspective. The Journal of pharmacology and experimental therapeutics, 370(3), 555–569. https://doi.org/10.1124/jpet.119.258707
(14) O’Mahony, C., Hilliard, L., Kosch, T., Bocchino, A., Sulas, E., Kenthao, A., … & Bared, G. (2017). Accuracy and feasibility of piezoelectric inkjet coating technology for applications in microneedle-based transdermal delivery. Microelectronic Engineering, 172, 19-25.
(15) Rad, Z. F., Prewett, P. D., & Davies, G. J. (2021). An overview of microneedle applications, materials, and fabrication methods. Beilstein Journal of Nanotechnology, 12(1), 1034-1046.
Turner, J. G., White, L. R., Estrela, P., & Leese, H. S. (2021). Hydrogel‐forming microneedles: current advancements and future trends. Macromolecular Bioscience, 21(2), 2000307.