Miniaturizing ELISA: How Precision Microdispensing Enables High-Sensitivity, Low-Volume Assays
Enzyme-linked Immunosorbent Assays (ELISAs) have long been the benchmark for detecting analytes in clinical and research settings. While effective, traditional ELISAs are resource-intensive, time-consuming to perform, and ill-suited for high-throughput, multiplex applications. As scientific workflows demand faster and more cost-efficient solutions, it is clear that the classic format needs a modern upgrade. Miniaturized ELISAs offer a powerful upgrade, leveraging picoliter dispensing to cut costs, accelerate timelines, and scale assays without sacrificing data quality. In this article, we discuss the shortcomings of traditional ELISA and how miniaturization paves the way for faster, scalable, and more efficient assays across diverse applications.
Why ELISA Needs an Upgrade
ELISA is the gold standard for immunodetection of target analytes and has been an enduringly valuable tool for both research and clinical applications. ELISA has proven particularly revolutionary in assessing infection status and remains a cornerstone of both routine diagnostics and cutting-edge research across various biomedical fields [1,2]. For example, healthcare professionals use it to measure levels of circulating antibodies against various pathogens, such as Hepatitis viruses and HIV, as well as for health screening purposes, like assessing prostate-specific antigen levels [3,4].
Despite its importance and ubiquity, this technique has limitations that render it a less viable option in modern workflows. It can be:
- Resource-intensive: They typically involve multiple steps (e.g., coating, blocking, washing, incubation), which take several hours and require qualified personnel for performance and interpretation.
- Reagent-intensive: They involve the use of expensive primary antibodies and potentially large volumes of different buffers and washing reagents when performed at large scales.
- Prone to variability: Lot-to-lot variation in antibodies or substrates can lead to inconsistent results over time. Cross-reactivity or non-specific binding can result in incorrect results. Improper washing or reagent issues can also affect accuracy. Furthermore, manual pipetting of ELISAs introduces additional challenges, such as increased risk of human error, underscoring the need for modern alternatives [5].
- Hard to mutiplex: Even at 96- and 384-well formats, multiplexing challenges can put a spanner in the works for teams looking to achieve resource efficiency while meeting competitive timelines.
The Case for Miniaturized ELISA
One solution that can address the problems of cost, throughput, and multiplexing is ELISA miniaturization. Thanks to technological advances, many assays and laboratory processes can now be scaled down, volume-wise, reducing the amount of reagents and sample required. A miniaturized ELISA can be performed using microarray or chip formats and scaled down to reaction volumes in the micro-, nano-, and picoliter ranges using a dedicated microdispenser. These capabilities bring several advantages to researchers, including:
- Conserving reagents: Miniaturization reduces reagent volumes per reaction, and precision microdispensers are designed to have low dead-volume, wasting less liquid between runs. This lowers overall consumable use and costs, freeing up budget for achieving greater throughput or addressing other experimental needs.
- Higher throughput: Microdispensing systems can rapidly process multiple microwells or microarrays, increasing throughput, so that researchers can process more samples in parallel. This facilitates large-scale studies, where accuracy isn’t impacted by inter-run variations.
- Faster workflows: Processing more samples at once shortens both research and clinical timelines, enabling faster clinical decision-making and potentially improving patient outcomes. Moreover, automated dispensers, and their possible integration with other robotic systems, reduce hands-on time, error, and variability across large sample sets [6].
- Multiplexing: Miniaturized ELISA platforms, including those that utilize picoliter dispensing, enable the simultaneous detection of multiple analytes within a single assay. This conserves sample, increases data density, and allows more comprehensive analysis in a single run.
- Better reproducibility: Precision microdispensers can deliver accurate, repeatable picoliter to microliter volumes of reagents, minimizing human error and variability. Uniform droplet deposition improves antigen/antibody binding consistency across wells or slides, leading to more reliable results.
For example, SCIENION’s sciFLEXARRAYER line enables ELISAs that would normally consume an entire 96-well plate to be performed within a single well, resulting in a more than 100-fold reduction in material consumption (Fig. 1).
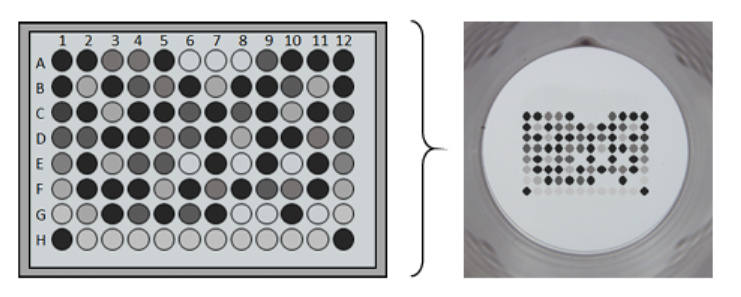
Figure 1. A sciFLEXARRAYER was used to produce a microarray ELISA with approximately 700 pL per spot, resulting in spots with a diameter of roughly 190 µm. Read the full application note to learn how this technology was used to assess cardiovascular disease biomarkers.
Precision Dispensing Challenges in Miniaturized Assays
Miniaturization relies on precision dispensing and is increasingly being adopted in clinical and research workflows. However, not all miniaturization technologies are created equal. While addressing the challenges of traditional ELISA, they also introduce new issues that can delay timelines or compromise data quality.
Inconsistent droplet volumes can cause variability across ELISA workflows. Adding too little or too much of a primary antibody affects antigen binding, resulting in variation, and possibly false negative/positive results. This inconsistency makes it difficult for researchers to draw reliable conclusions or reproduce results. Additionally, poor spot alignment and irregular spot morphology can create similar issues. Some dispensing technologies also rely on contact-based methods, which carry a higher risk of cross-contamination and issues with spot morphology.
These limitations can leave researchers back where they started, forcing them to confront the same challenges as traditional, non-miniaturized manual methods, and wasting valuable resources and generating inconsistent, unreliable data.
SCIENION Technology for Effective ELISA Miniaturization
SCIENION’s sciFLEXARRAYER instruments help overcome many of the ELISA challenges by enabling highly accurate, non-contact dispensing of volumes as small as 10 picoliters. These platforms also offer the flexibility to adapt experimental approaches to varying scales and multiplexing requirements. This technology ensures consistent microdispensing, meaning volumes and spot morphologies are consistent within and across runs.
Furthermore, SCIENION technology achieves zero dead volume and utilizes non-contact dispensing, enabling researchers to preserve expensive reagents and prevent cross-contamination (Fig. 2). Moreover, sciFLEXARRAYER comes in a full range of instruments from models designed for research and methodological development (e.g., sciFLEXARRAYER S3) to fully integrated systems for high-throughput manufacturing (sciFLEXARRAYER S100). This scalability allows laboratories and production facilities alike to benefit from the precision and reliability of SCIENION’s dispensing technology.

Figure 2. SCIENION instruments utilize piezo-acoustic dispensing of ultra-low volumes, down to 10 pL, and achieve absolute zero dead volume and precision positioning for accurate and multiplexed ELISA.
Use Cases & Applications
Backed by a robust microdispenser, researchers can unlock entire vistas of potential applications while boosting the speed and efficiency of their existing ELISA workflows. Applications that benefit from ELISA miniaturization include:
- Multiplex Cytokine Profiling: Miniaturized ELISAs enable the simultaneous measurement of numerous cytokines from small sample volumes, allowing for comprehensive immune profiling and accelerating the identification of inflammatory markers to inform clinical decision-making and unlock new therapeutic approaches.
- Infectious Disease Panels: Miniaturized ELISAs can be used to detect antibodies for multiple pathogens in a single test, streamlining infectious disease diagnostics by conserving samples and significantly reducing turnaround time. Miniaturization can be especially valuable in complex cases with limited sample availability [7].
- Biomarker Discovery and Validation: By enabling assay multiplexing, miniaturization can achieve rapid screening of candidate biomarkers from clinical samples. Without a precision dispenser, applications like this would be prohibitively expensive and likely consume too much sample to be viable.
Conclusion
Miniaturized ELISA platforms are redefining what’s possible in diagnostics and research. With precise, non-contact dispensing platforms like SCIENION’s sciFLEXARRAYER, scientists can overcome traditional ELISA limitations while unlocking high-throughput, multiplexed, and sample-efficient workflows. These advancements reduce timelines and conserve precious reagents, enabling advancements in diagnosis and discovery applications. ELISA miniaturization helps alleviate the perennial pressure for higher efficiency without compromising sensitivity or data quality.
Talk with one of our experts to learn more about how SCIENION can help you scale multiplexed immunoassays.
References
- Ho A, Galgut O, Faustini S, Peters N, Shields A, Klenerman P, et al. Implications of suboptimal measles immunity in UK health-care workers. Lancet. 2024 Jul 6;404(10447):23–4.
- Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015 Oct;72:4–15.
- Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010 Jun;28(6):595–9.
- Ly TD, Laperche S, Couroucé AM. Early detection of human immunodeficiency virus infection using third- and fourth-generation screening assays. Eur J Clin Microbiol Infect Dis. 2001 Feb;20(2):104–10.
- Lippi G, Lima-Oliveira G, Brocco G, Bassi A, Salvagno GL. Estimating the intra- and inter-individual imprecision of manual pipetting. Clinical Chemistry and Laboratory Medicine (CCLM) [Internet]. 2017 Jan 27 [cited 2024 Sep 2];55(7). Available from: https://www.degruyter.com/document/doi/10.1515/cclm-2016-0810/html
- Holland I, Davies JA. Automation in the Life Science Research Laboratory. Front Bioeng Biotechnol. 2020;8(571777).
- Siavashy S, Soltani M, Rahimi S, Hosseinali M, Guilandokht Z, Raahemifar K. Recent advancements in microfluidic-based biosensors for detection of genes and proteins: Applications and techniques. Biosensors and Bioelectronics: X. 2024 Aug;19:100489.