Cell Line Development
Cellenion and Miltenyi Biotech have collaborated to demonstrate the complementarity of the cellenONE X1 single-cell deposition unit and MACSQuant Tyto Cell Sorter for cell line development. The results were published in a joint application note which was presented for the first time at the BioProcess International European Summit 2018 in Amsterdam, April 23-25.
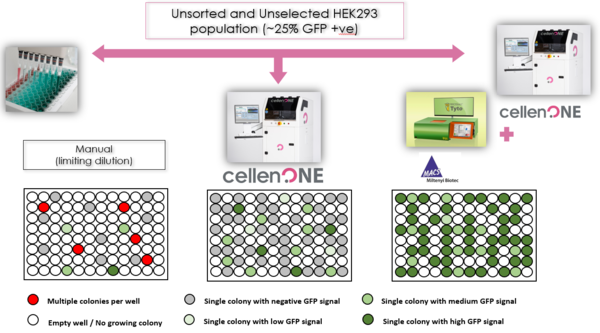
Proof-of-principle studies were performed with a suspension of HEK293T wild type cells and stably transduced HEK293T cells expressing a protein (GFP, green fluorescent protein). HEK293T is a cell line derived from human embryonic kidney cells which is commonly used to produce therapeutic proteins. The study focused on a comparison between manual cloning, automated cloning by cellenONE X1 and automated sorting by MACSQuant Tyto with subsequent cloning by cellenONE X1. Results clearly demonstrated the power of combining the use of MACSQuant Tyto and cellenONE X1 with respect to efficiency, time, simplicity of operation and cost-savings.
cellenONE X1
cellenONE is a product developed by Cellenion.
For more information, visit the official Cellenion website: www.cellenion.com
“We have been using cellenONE in combination with our latest cell sorter, the MACSQuant Tyto. These two technologies offer a great combination for cell line development. The MACSQuant Tyto Sorter is an easy to operate instrument that gently enriches the cell population of interest, while cellenONE’s accuracy allows for highly efficient clone selection from the enriched population. Used together, these two technologies reduced our monoclonal antibody development protocols by nearly 4 weeks.”
Bastian Ackermann,
Marketing Manager Flow Sorting & Imaging at Miltenyi Biotech
“Our cellenONE X1 allows for unique optically monitored single cell isolation and thus outpaces any manual methods for this purpose. Combining cell sorting with MACSQuant Tyto and cloning with cellenONE X1 provides superior benefits for advanced cell line development: Beside their accuracy, both systems handle cells very gently and which results in high cell viability. Furthermore, both systems are fully automated and thereby facilitate laboratory routine.”
Guilhem Tourniaire,
Managing Director of Cellenion SASU



